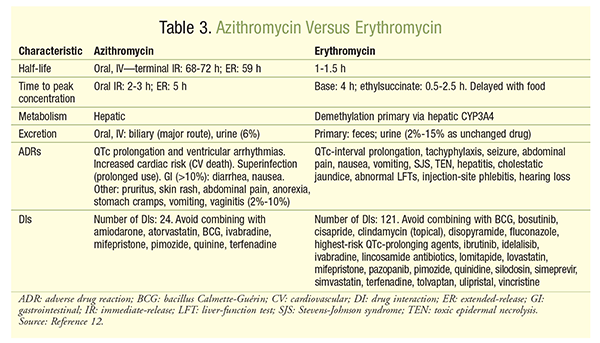
Azithromycin Vs. Clarithromycin For Mac
This multicenter, randomized, dose-ranging study was performed to determine the safety and efficacy of two different doses of azithromycin for treating disseminated Mycobacterium avium complex (MAC) in patients with AIDS. Eighty-eight AIDS patients with symptoms and blood cultures consistent with disseminated MAC were treated with 600 or 1,200 mg of azithromycin daily for 6 weeks; 62 patients completed the entire 6 weeks of study. Of note, this study was done prior to the time when combination antiretroviral or anti-MAC regimens were the standard of care. Over the 6-week study period, symptomatic improvement was noted in both dose groups. Microbiological responses were comparable, with mean decreases of 1.5 and 2.0 log CFU/ml in the high- and low-dose groups, respectively.
Sterilization of blood cultures occurred in 54% of samples; patients with lower baseline colony counts were more likely to achieve culture negativity. Resistance developed in one patient.
Who also had disseminated Mycobacterium avium complex received either azithromycin 250 mg every day, azithromycin 600 mg every day, or clarithromycin 500 mg twice a day, each combined with.
Gastrointestinal symptoms were the most common side effects and were more frequent in patients receiving 1,200 mg. Azithromycin is a useful alternative treatment for disseminated MAC infection in AIDS patients. Symptomatic improvement correlates with measurable decreases in mycobacterial load. The widespread use of chemoprophylaxis and the availability of potent antiretroviral therapies have resulted in an overall decrease in the incidence of serious opportunistic infections. Mycobacterium avium complex (MAC), however, still may cause serious disease, either with classical systemic manifestations or as a focal inflammatory lymphadenitis occurring shortly after the initiation of protease inhibitor-containing regimens (, ). Good activity against MAC has been demonstrated both in vitro and in vivo in the beige mouse model for both clarithromycin and azithromycin , and there are a number of studies supporting the utility of macrolide-containing regimens as treatment or prophylaxis for MAC infection (–, ).
Clarithromycin has been the macrolide used in most of these studies, based on its clinical efficacy, which has been established in monotherapy trials (, ). The concern over treating mycobacterial disease with monotherapy was underscored, however, by the noted fourfold or higher increases in MICs, with recrudescence of symptoms in 21% of patients. Azithromycin has a favorable pharmacokinetic profile , possibly less potential for allowing organisms to develop resistance , and few significant drug interactions. Additionally, its efficacy as a prophylactic agent against MAC has been demonstrated , and it is now considered the standard of care. Nonetheless, azithromycin has been used less commonly as the primary macrolide for treatment of MAC, due to limited published data from clinical trials; one small pilot study showed symptomatic improvement and decreases in quantitative mycobacteremia in patients treated with a daily dose of 500 mg of azithromycin for 10, 20, or 30 days.
This short-duration, dose-ranging, monotherapy study in AIDS patients with disseminated MAC infection was completed prior to the availability of predictably active anti-MAC regimens, but serves to establish the efficacy of azithromycin as an alternative primary treatment for disseminated MAC. We acknowledge that the current standard of care requires combination regimens for treating active MAC disease. MATERIALS AND METHODS Human immunodeficiency virus-infected adults, at least 18 years of age, with symptoms consistent with disseminated MAC and with blood cultures from which MAC had been isolated within 4 weeks prior to enrollment, were eligible to participate in this study. Patients were excluded from the study if they were pregnant or if they had received treatment with any investigational drug, immunostimulant, or any drug with known activity against MAC within 7 days of enrollment. All patients gave written informed consent according to the Institutional Review Boards of the participating clinical centers.
Patients who met all eligibility criteria were randomized to receive either 600 or 1,200 mg of oral azithromycin daily for 6 weeks. Clinical and microbiological evaluations and safety-monitoring laboratory tests were performed at baseline and weeks 1, 3, and 6. Symptomatic responses to treatment were evaluated by both patients and investigators. Patients completed questionnaires at baseline and subsequent evaluation periods that addressed specific symptoms, including fever, night sweats, nausea, vomiting, cough, abdominal pain, anorexia, and fatigue. Investigators’ assessments of clinical response at weeks 1, 3, and 6 used a 4-point scale: complete response, partial response, no response, and disease progression.
Overall assessment of response at the end of therapy used a 7-point scale: marked, moderate, or slight deterioration; no change; or slight, moderate, or marked improvement. At the end of the study period, patients with any improvement of their symptoms were allowed to continue on azithromycin with the option of adding other drugs with anti-MAC activity. Quantitative blood cultures for mycobacteria were performed with the Isolator tube (Wampole Laboratories) lysis-centrifugation culture system. Susceptibility testing of isolates with azithromycin was performed by a broth dilution radiometric method. Discrete MICs were defined by using radiometric growth data and recorded as the MIC for 99% of isolates tested/MIC for 99.9% of isolates tested (MIC 99/MIC 99.9). Analyses of efficacy were performed within each of the 600- and 1,200-mg treatment groups as well as between the groups (600 versus 1,200 mg). For dichotomous endpoints, such as sterilization of blood cultures, positive bacterial response, complete clinical response, and complete response for specific signs and symptoms, 95% confidence intervals were constructed from the normal approximation with a continuity correction, and comparisons were made with the Cochran-Mantel-Haenszel test adjusted for the center.
For continuous endpoints, such as colony count, clinical response, and specific signs and symptoms, a mixed-effects model was used, adjusting for center, baseline, treatment, time, and treatment-by-time interaction in the model. From this model, results were reported for each time point, and 95% confidence intervals were constructed from least-square means and their standard errors. All P values are unadjusted and from two-tailed tests. RESULTS Eighty-nine patients were randomized at eight clinical centers to receive either 600 or 1,200 mg of azithromycin daily for 6 weeks.
Eighty-eight patients received treatment, and 62 patients completed the entire 6 weeks of the study. The groups were comparable, and demographic and baseline clinical characteristics are shown in Table. Concurrent illnesses and concomitant medications were also similar in both groups (data not shown).
Of note, this study was completed prior to the availability of potent antiretroviral regimens. Clinical efficacies, as defined by both objective and subjective symptomatic responses to therapy, were similar in both treatment groups. At the end of 6 weeks of therapy, the majority of both patients and investigators reported at least slight improvement in symptoms related to disseminated MAC, although there was some variance in degree. Microbiological efficacy was assessed by analysis of reduction of MAC colony counts. A total of 56 patients had quantitative blood cultures obtained at baseline and at 3 and/or 6 weeks. Comparable results were seen with both dosages with decreases of between 1.5 and 2.0 log CFU/ml, respectively, in the high- and low-dose groups (Fig.
Sterilization of blood cultures occurred in 30 of the 56 patients (54%) without significant differences between the two treatment groups. Patients with lower baseline colony counts were more likely to achieve culture negativity (Fig. Only one patient in the 600-mg treatment group had a significant rise in colony counts at 6 weeks following initial clinical and microbiological responses; this was correlated with a significant (4-fold) increase in MIC, but not with a recrudescence of symptoms.
Baseline MICs (recorded as MIC 99/MIC 99.9) were determined for 63 isolates and ranged from 3.3/5.0 to 128/128 μg/ml (median, 15.0/21.9). There was no difference between the 600- and 1,200-mg treatment groups, nor did the baseline MIC have a significant impact on microbiological response, including sterilization of blood cultures. Relationship between degree of mycobacteremia at baseline and subsequent sterilization of blood cultures in patients responding to treatment with azithromycin. Twenty-six patients (12 assigned to the 600-mg dose and 14 to the 1,200-mg dose) prematurely discontinued treatment with azithromycin, with the majority of discontinuations not related specifically to the effects of the study drug.
Seventeen patients (9 taking 600 mg and 8 taking 1,200 mg) received ≤21 days of therapy. Four patients in each group prematurely discontinued for reasons related to the study drug. In the 600-mg group, side effects resulting in early discontinuation included those for two patients with rash and two patients with nausea and vomiting. In the 1,200-mg group, two patients stopped taking the azithromycin for similar gastrointestinal complaints, and two patients had progression of their MAC disease, for which one resulted in death. Four patients assigned to the 1,200-mg arm were excluded from the final analysis because they began receiving other agents with potential activity against MAC, but in two instances, this occurred during the last week of study. Treatment-related side effects were reported in 24 (61%) patients taking 600 mg daily and in 37 (79%) of those taking 1,200 mg. Gastrointestinal complaints, particularly abdominal pain, nausea, vomiting, and diarrhea, were the most common side effects, occurring in 38 and 64% of the low- and high-dose groups, respectively.
These complaints were primarily mild in the lower-dose group, but abdominal pain and diarrhea were more often moderate to severe in the higher-dose group. Other commonly noted side effects included transient sensorineural hearing loss in a total of 10 patients and rash, which occurred in 2 patients in each group. No deaths were directly attributable to azithromycin therapy, although two patients in each group died during the 6-week study period, one in each study arm within the first 2 weeks, and one in each study arm after at least 4 weeks of therapy. DISCUSSION Untreated disseminated MAC in AIDS patients has a uniformly dismal prognosis, with significant morbidity and shortened survival, but timely diagnosis and specific macrolide-containing regimens have had a dramatic impact on MAC disease.
This short-duration azithromycin dose-ranging study corroborates previous experience with both azithromycin and clarithromycin as treatment for disseminated MAC in AIDS patients (–, –). Symptomatic improvement was generally coincident with microbiological response, and side effects were not particularly severe. There was no significant difference in efficacy between the 600- and 1,200-mg daily doses, but the latter did tend to have more gastrointestinal side effects. The patients most likely to sterilize their blood cultures were those who had low baseline colony counts, regardless of measurable susceptibility to azithromycin.
There has been dissenting opinion, however, regarding the microbiological efficacy of azithromycin. have recently published a 16-week study comparing two treatment regimens: 600 mg of azithromycin daily plus 800 to 1,200 mg of ethambutol daily versus 500 mg of clarithromycin twice daily plus 800 to 1,200 mg of ethambutol daily. The clinical responses were similar in the two treatment groups, and there were no significant differences when comparing median levels of bacteremia at individual study visits. In clarithromycin-treated patients, however, the estimated median time to clearance of bacteremia was more rapid, and there was a higher proportion who had sterile blood cultures at weeks 8 and 16 (but not at week 12).
The conclusion was that clarithromycin has superior microbiological efficacy against MAC. The argument against the accuracy of that conclusion is sample size. Assessment of comparative treatment effects can only be done fairly if competing risks either are accounted for and stratified, or if the sample size is large enough to negate their influence. The number of evaluable patients at baseline was small (azithromycin, n = 16; clarithromycin, n = 21), and by week 16 was reduced by approximately one-third. With such a small study population, factors that could influence outcome (such as concurrent illnesses, concomitant medications, and potential drug interactions) cannot be adequately assessed.
Such factors, rather than true biological or pharmacological phenomena, are a more probable explanation for the differences observed between the study by Ward et al. and our study. Despite the demonstrated efficacy of both clarithromycin and azithromycin against MAC, prolonged monotherapy always raises concerns about the potential development of resistance, a known drawback of monotherapy of other mycobacterial diseases. In the study by Chaisson et al. , virtually all patients had baseline isolates that were susceptible to clarithromycin, but resistance developed in 21% of the patients during the first 12 weeks of therapy, occurring sooner in patients with higher baseline bacterial counts. In our 6-week study, high MICs for baseline isolates were not necessarily correlated with clinically unresponsive disease, and microbiological resistance emerged in only one patient. Perhaps of even greater concern is that cross-resistance between azithromycin and clarithromycin has been reported in vitro and in vivo resistance to clarithromycin has been reported both in the beige mouse model and in patients, even when used in combination with other antimycobacterial drugs.
A recent study utilizing the beige mouse model showed that resistance emerged more frequently following treatment with clarithromycin compared to azithromycin. Disseminated MAC is treated most effectively with a combination therapy regimen that contains a macrolide. This study demonstrates that azithromycin is useful as the primary macrolide in the treatment of disseminated MAC. Symptomatic improvement and quantitative reduction in mycobacteremia occurred with both 600- and 1,200-mg doses. The former is better tolerated, making it the likely dose for combination regimens. Few drug interactions and theoretical advantages in resistance patterns make azithromycin a reasonable alternative to clarithromycin in a primary MAC treatment regimen. Issues regarding resistance and cross-resistance, as well as appropriate alternatives for breakthrough disease, must be addressed in future studies.
MAC lung infections are a growing public health problem. The ATS / IDSA 2007 guidelines for the treatment of these non-tuberculous mycobacterial infections recommend the use of a macrolide or azalide (clarithromycin or azithromycin), rifampicin or rifabutin and ethambutol. For MAC disseminated infections, several studies have compared combinations containing clarithromycin or azithromycin and found no significant difference in efficacy. No randomized controlled trials have been performed for pulmonary infections to compare clarithromycin and azithromycin in terms of efficacy. Clarithromycin is often used as a first-line treatment in France, but its tolerance is often poor, particularly in terms of risk of hepatitis, metallic taste in the mouth, nausea or vomiting, and it interacts with many drugs via cytochrome p450. In particular, it increases the toxicity of rifabutin, in particular in terms of uveitis. Azithromycin has fewer side effects especially less digestive toxicity and drug interactions than clarithromycin.
The hypothesis is therefore that the efficacy of azithromycin would be non-inferior in comparison with that of clarithromycin. Condition or disease Intervention/treatment Phase Lung Infection Mycobacterium Avium Complex Drug: Clarithromycin 1000 MG Drug: Azithromycin 250 mg Drug: Rifampicin Drug: Ethambutol Phase 3. Three spontaneous sputum specimens will be obtained at baseline and 6 months. If it's not possible to obtain good quality specimens, the clinician may perform three hypertonic aerosol-induced sputum specimens on 3 consecutive days.
In the absence of sputum (spontaneous or induced), bronchoscopic aspiration and two post-bronchoscopy sputum specimens will be performed. Microscopic examination will be performed after auramine or Ziehl-Neelsen stain.
Specimens will be cultured at 37°C after decontamination on Lowenstein-Jensen solid medium and Coletsos medium. Results will be expressed quantitatively as the number of colonies per tube according to a logarithmic scale.
Culture on liquid medium will also be performed. The analysis will be based on the Mycobacteria National Reference Centre microscopy, culture and antibiotic susceptibility test results.
Chest x-ray and chest high-resolution CT will be realised at baseline and at 6 months. Chest x-ray will be described according to:. type of lesion: opacity/solitary pulmonary nodule, excavated opacity/ fibrocavitary disease, interstitial syndrome (multiple nodule, dense airspace disease). precise site (upper lobe, right middle or lower lobe, left lower or upper lobe) Chest CT will be described according to:. type of lesion: nodules, micronodules, bronchiectasis, mass, interstitial syndrome (and its type). precise site of the segment(s) involved. number of lesions.
Clarithromycin To Azithromycin Conver…
dimensions of the various lesions (long and short axes) and volume, whenever possible. Radiological improvement Time Frame: 12 months. Chest x-ray and chest high-resolution CT will be realised at baseline and at 12 months.
Chest x-ray will be described according to:. type of lesion: opacity/solitary pulmonary nodule, excavated opacity/ fibrocavitary disease, interstitial syndrome (multiple nodule, dense airspace disease). precise site (upper lobe, right middle or lower lobe, left lower or upper lobe) Chest CT will be described according to:. type of lesion: nodules, micronodules, bronchiectasis, mass, interstitial syndrome (and its type). precise site of the segment(s) involved. number of lesions.
dimensions of the various lesions (long and short axes) and volume, whenever possible. Sputum conversion (culture results of respiratory specimens) Time Frame: 3 months.